Second Scientific Lecture-Course:
Warmth Course
GA 321
Lecture VI
6 March 1920, Stuttgart
My dear friends,
We will today first examine a phenomenon that comes in the region where heat, pressure and the expansion of bodies are related. You will see that by a simultaneous examination of the things we experience in this field the way will open to an understanding of what heat really is. First we will turn our attention to what is revealed here in these three tubes. In the first one on the right, we have mercury in a barometer tube and on top of it is some water. Water placed in such a manner in this space evaporates. The water is in a vacuum, as we call it, in empty space, and it can be stated that the water evaporated. The small amount of water in the tube gives off vapor. We can determine that it evaporates by testing for the presence of water vapor in the space above the mercury. When you compare the height of the mercury column in this tube with the height here where the mercury is under the normal atmospheric pressure, and where there is no water vapor over the mercury, you will see that the level is lower in the tube containing water (Fig. 1a, 1b). Naturally, the mercury can lower only if there is a pressure on top of the column. For in the barometer tube, there is no pressure on the top of the column. There is only empty space and the mercury column balances the atmospheric pressure and is equal to if. Here it is forced down. When we measure we find the value of this difference in height. And the amount of the depression is brought about by the pressure of the water vapor, by the vapor tension as it is called. That is, the mercury volume is forced down here. We see therefore, that vapor always presses on the confining walls. Moreover, a definite pressure corresponds to a definite temperature. We can demonstrate this by warming the upper part of the tube. You can see that when the temperature is raised, the mercury column sinks, due to the increased pressure of the vapor. Thus we see that the vapor increases its pressure on the wall more and more the higher its temperature. You can observe the mercury fall and see how the vapor tension increases with the temperature. The volume occupied by the vapor is correspondingly increased.

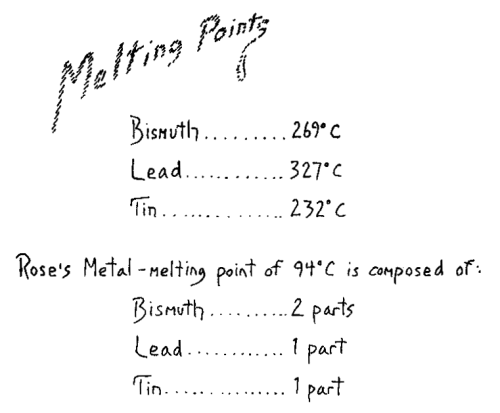
In the second tube we have alcohol over the column of mercury (Fig. 1c). Again you can see the liquid alcohol occupying definite volume. It evaporates and consequently the column is less in height than the barometric column on the left. If I measure, I find that it is shorter than the column which is under the pressure of the water vapor. We must wait until the water vapor returns to the same temperature as it was before being heated. Then we will find the vapor tension dependent on the substance we are using. The tension is greater in the case of alcohol than in the case of water. Here again, I can make the same experiment with heat. You will see that the pressure becomes considerably greater when we raise the temperature. When we cool the vapor to the same point at which it was at first, the mercury column rises, since with smaller vapor tension there is less pressure.
In the third tube we have ether under the same conditions as in the other tubes. It also evaporated (Fig. 1d). You observe the column here is very low. From this you can see that ether evaporating under the same conditions as water shows a widely different pressure. Not only is the pressure exerted by a vapor dependent on the temperature, but on the material as well. Here you see the effect of increased temperature, but on the material as well. Here you see the effect of increased temperature, shown by lowering of the column (tube warmed slightly) due to the rise in vapor pressure. We can again in this case, verify the phenomena and thus round out our survey and lead to the result we wish to attain.
Now there is an occurrence that I wish especially to call to your attention. You know from the foregoing observation and also from elementary physics that solids may be changed to liquids and liquids to solids if we raise the temperature above the melting point and lower it below the melting point. Now, when a fluid body is solidified by being brought under the melting point, it remains a solid body. The noteworthy fact, however, is that if we impose on this solid body a sufficiently great pressure, it will melt at a temperature below its melting point under ordinary pressure.
Thus it can become liquid at a lower temperature than the one at which it solidified. You know that water changed to ice at 0°C. and it must be a solid at all temperatures under 0°C. We will now carry out an experiment on this ice which will show you that we can make it a liquid without raising the temperature. Ordinarily, we would have to raise the temperature to do this. In this case we will not raise the temperature but simply exert a strong pressure on the ice. This we can do by hanging a weight over the ice by means of a thin wire. The ice melts under the wire, and the wire cuts its way through the ice. Now, you would expect this block of ice to fall apart into two pieces since it is being cut through the middle. It we could make it work faster you would see the results of this experiment. (Note: the cutting of the block proceeded so slowly that the result described in the following did not occur until several hours after the end of the lecture.) If you will now step up here and examine the block of ice, you will find there is no reason to fear that the two halves will crash down when the wire has cut its way through. For the solid ice grows together at once above the cut; so that the wire goes through the block, the weight falls off and the block remains whole. This shows that fluidity is brought about under the pressure of the wire, but as soon as the fluid is released from the spot where the pressure is exerted, it solidifies and the block of ice becomes whole again.
At the temperature of ice, the state of fluidity only establishes itself under increased pressure. Thus a solid can be melted at a temperature under its melting point, but the pressure must be maintained if it is to stay melted. As soon as the pressure is released it reverts to the solid state. This is what you would see if you could wait here an hour or so.
A third thing I wish to present to you and which will furnish support for our observations is the following: To illustrate it we can take any bodies making an alloy, that is, mixing without forming a chemical compound; the principle holds for all of them. In this tube we have bismuth that melts at 269°C. and here we have tin, melting at 232°C. Thus we have three bodies all of which have melting points over 200°C. Now we will first melt these three, bringing them into the fluid condition in order to form an alloy. They will mix without combining chemically. (Note: the three metals were melted and poured together.)
Now, you would naturally reason as follows: Since each of these metals has a melting point above 200°C. it would remain solid in boiling water, for water has a melting point of 0°C. and a boiling point of 100°C. Therefore these three metals could not melt in boiling water. Let us however carry out the experiment of bringing the allow, the mixture of the three, into water, just at the boiling point of 100°C. In this way we can see how it acts. We hold the thermometer here in the fluid metallic mixture and read a temperature of 94°C. This shows that although no single metal was fluid at this temperature, the alloy is fluid. We can state the fact thus: when metals are mixed, the fact is brought out that the melting point of the mixture is lower than the melting point of any of its constituents. Thus you can see how bodies mutually influence each other. From this particular fact we can derive an important principle for our view of the nature of heat phenomena.
Here we have the still fluid alloy in boiling water that is at 100°C., and now we let the water cool, observing the temperature meanwhile. The alloy finally solidifies. By measuring the temperature of the water at this point, we have the melting point of the alloy and can show that this melting point is lower than the melting point of any of the single metals.
We have now added this phenomenon to the others to extend the foundations of our view. Let us continue by tying in the things we considered yesterday in regard to the distinction between the solid, the fluid and the gaseous or vapor states. You know that solid bodies such as most metals and other mineral bodies, occur not in an indefinite form, but in very definite shapes that we call crystals. We can say: Under ordinary circumstances as they exist on the earth, solids occur in very definite shapes or crystal forms. This naturally leads us to turn our attention to these forms, and to try to puzzle out how these crystals originate. What forces lie at the foundation of crystal formation? In order to gain some insight into these matters, it will be necessary for us to consider the forces on and around the earth in their entirety as they are related to solids.
You know that when we hold a solid in our hand and let go of it, it falls to the earth. In physics this is usually explained as follows: The earth attracts solid bodies, exerts a force on them; under the influence of this force—the gravitational force—the body falls to the earth.
When we have a fluid and cool it so that it solidifies, if forms definite crystals.
The question is now, that is the relation between the force acting on all solids—gravitation—to these forces tending to produce crystal form which must be present and active to a certain extent? You might easily think that gravity as such, through whose agency a body falls to the earth (we may at this stage speak of the force of gravity) you might think that this gravitational force had nothing to do with the building of crystal form. For gravity affects all crystals. No matter what form an object may have, it is subject to gravity. We find when we have a number of solids in a row and take way the support, that they all fall to earth in parallel lines. This fall may be represented in somewhat the following way: (Fig. 3).
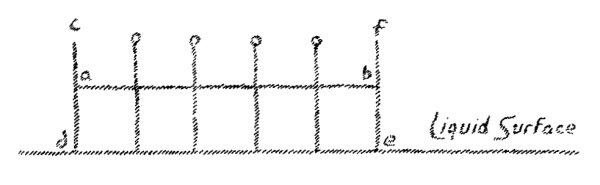
We can say, whatever form a solid may have, it falls along a line perpendicular to the surface of the earth. When now, we draw the perpendicular to these parallel lines of fall, we obtain a surface parallel to the earth's surface (line a-b, Fig. 3). By drawing all possible perpendiculars, to the lines of fall, we will obtain a complete surface parallel to the earth's surface. This is at first an imagined surface. We may now ask the question, where in reality is this surface? It is actually present in fluid bodies. A liquid which I place in a vessel shows as a real liquid surface that which I have assumed here as produced by drawing perpendiculars to the line of fall (see c, d, e, f, in Fig. 3).
What is really involved here and what does it mean? What we are speaking of is a thing of tremendous import. For, imagine to yourselves the following: Suppose someone were trying to explain the liquid surface and stated it this way. Every minute portion of the liquid has the tendency to fall to the earth. Since the other portions hinder this, the liquid surface is formed. The forces are really there, and the presence of the liquid causes the surface to form.
Picture to ourselves the real condition of the bodies you are going to let fall, and nature herself will show you what you have said in this explanation, (Fig. 4). You must include the liquid surface in your thinking. I have said formerly: the liquid surface is to be thought of in its relation to solids at right angles to their line of fall. When you think this through to the end, you come upon the noteworthy thing that what you have to bring into the solid as something thought out, this is represented in a material way before you by liquid bodies. These incorporate, as it were, what is materially present in the liquid. We may say: bodies of lower degrees of aggregation, solids in their relation to the earth, show a picture of that which is really present in the liquid, in a material way, and which in the case of water present in the liquid, in a material way, and which in the case of water prevents the surface particles from falling into the liquid. This is pictured, as it were, in considering the solid in its relation to the whole earth.
Think what this enables us to do When I draw the line of fall and the surface formed under the pressure of a system of falling bodies, then I have a picture of the gravitational activity. This is a direct representation of matter in the liquid state.
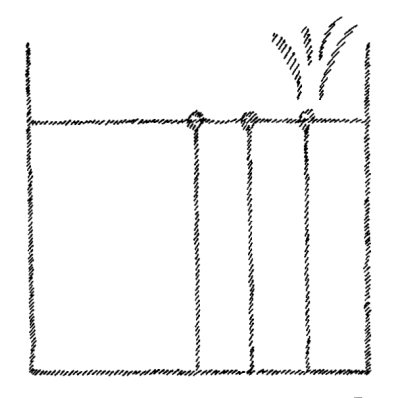
We can proceed further. When we leave water at any temperature sufficiently long it dries up. Water is always evaporating. The conditions under which it forms a liquid surface are only relative. It must be confined all around except on the liquid surface. It evaporates continuously, more rapidly in a vacuum. If we draw lines showing the direction in which the water is tending, their direction must indicate the movement of the water particles when it actually evaporates. When I actually draw these lines, however, I get nothing more or less than a representation of a gas that is enclosed all around and is striving to escape in every direction (Fig. 5). On the surface of water there is a certain tendency which, when I picture it for explanatory purposes, represents a gas set free and distributing itself in all directions. So again, we can state the proposition: that which we observe in water as a force is actually represented in a material way in a gas.
There is a curious fact brought out here. If we look at fluids correctly from a certain point of view, we discover in them a picture of the gaseous state of aggregation. When we picture solids properly, we discover in them a representation of the fluid state of aggregation. In every step as we go down there is a representation of the preceding step. Let us illustrate by going from below up. We can say, in the solids we have a representation of the fluid state, in the fluid a representation of the gaseous, in the gaseous a representation of heat. It is this that we have especially to deal with tomorrow. I will say only this today, that we have sought to find the bridge for thought from gases to heat. It will become clearer tomorrow. Now when we have followed further this path of thinking:
In solids the picture of the fluid state;
In fluids the picture of the gaseous state;
In gases the picture of the heat state;
Then we will have, indeed, taken a great step ahead. We have advanced to the point where we have a picture in the gaseous state which is accessible to human observation, of heat manifestations and even of the real nature of heat itself. The possibility then exists for us that by rightly seeking the representations of heat in the gaseous state, we can explain its nature even though we are obliged to admit that it is an unknown entity to us at the outset. But we must do this in a proper manner. When the various phenomena that we have described so far are handled as physics usually handles them, we get nowhere. But when we hold correctly in our minds those things that are revealed to us by bodies under the influence of heat and pressure, then we will see how we, actually in fact, come to stand before that which the gases can reveal to us—the real being of heat.
In cooling, where we deal with the liquid and solid states, the being of heat penetrates further. We have then to recognize in these states the nature of this entity, although we can do it best in the gaseous condition where it is more evident. We must see whether in the fluid and solid states, heat suffers a special change, and thus work out the distinction between the manifestation in the gas where it shows itself in pictures form and its manifestation in fluids and solids.

